CATALYST FOR A CURE
The Catalyst for a Cure Vision Restoration Initiative, founded by the Glaucoma Research Foundation, brought together four scientists from prestigious academic centers chosen for their expertise in retinal ganglion cell restoration, replacement or repair, neuroprotection, and clinical ophthalmology.
When you donate to Cure Glaucoma Foundation, some of those funds are used to support this important research program because we believe in their goal to work collaboratively to find novel ways to restore vision lost due to glaucoma by focusing on ways to restore, repair, and rebuild the optic nerve. Once successful, the impact of the work by Catalyst for a Cure will be profound as currently there are no effective therapies to restore vision.
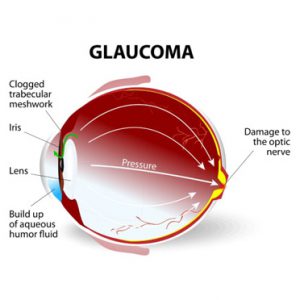
There are over a million retinal ganglion cells (RGCs) in the human retina, and they allow you to see as they send the image to your brain. Unfortunately, once RGCs die in glaucoma, they are not replaced, leading to vision loss. In addition to creating specialized biological tools to identify the different types of RGCs, the team is developing a method for transplanting RGCs and examining the role of genetic signals that influence RGC development and survival.
The Catalyst for the Cure (CFC) Vision Restoration Initiative is pursuing two major goals:
- Developing a strategy for optic nerve cell transplantation
- Developing neuroprotective therapies for glaucoma.
For the latter, the team hopes to use such therapies to improve the function of injured-but-not-yet-dead optic nerve cells, improve the survival of transplanted optic nerve cells, and halt the progression of vision loss from glaucoma.
In the past year, there has been significant progress on both fronts. A major barrier to optic nerve cell transplantation is a tissue called the inner limiting membrane (ILM). The ILM prevents the injected cells from moving out of the vitreous (where they are injected) into the retina (where they need to integrate with other retinal cells). The CFC team has developed a surgical technique to remove part of the ILM and are currently testing whether this improves optic nerve cell transplantation. In addition, the team has developed an imaging technique to test whether the transplanted cells are making useful connections with the rest of the retina.
The team conducted a high-throughput screening looking for chemicals that increase survival and increase (not decrease) connectivity with other nerve cells. Artificial intelligence was then used to mine the results and identify a potential drug target, called “GCK-IV kinases.” The team showed that strategies that target GCK-IV kinases protect optic nerve cells in the retina as well as those derived from stem cells, improving prospects for transplantation. These results were published in the December 2020 issue of Proceedings of the National Academy of Sciences.
In order to identify novel neuroprotective targets for glaucoma, the team measured the change in expression of every gene in the genome. The CFC collaboration is using molecular scissors called CRISPRs in order to delete candidate genes and test their role in a model of glaucoma developed by one of the investigators.
Finally, it is well known that optic nerve cells are not all the same and that subtypes exist. Using the same model of glaucoma mentioned above, the CFC investigators established that certain subtypes were more resistant to glaucoma while others were more sensitive. This seemed to correlate with the expression of a protein called osteopontin (i.e. more resistant cells had higher levels). To confirm the hypothesis that osteopontin was responsible for protecting optic nerve cells, the team added osteopontin to the retina and showed that susceptible cells became resistant. Conversely, if they removed osteopontin, resistant cells became more sensitive. Finally, they showed that osteopontin might be a potential biomarker of glaucoma.
Taken together, the team has several exciting candidate targets to improve optic nerve cell survival. The CFC team is now planning next steps to translate these techniques to the clinic and working on improving optic nerve cell transplantation.

Xin Duan, PhD
Assistant Professor, Department of Ophthalmology and Physiology
Weill Institute for Neurosciences
University of California, San Francisco
Dr. Duan’s laboratory investigates retinal ganglion cells subtype-intrinsic factors and tests their roles in optic nerve regeneration and vision recovery.

Yang Hu, MD, PhD
Assistant Professor, Department of Ophthalmology
Stanford University School of Medicine
Stanford, CA
The Hu laboratory focuses on the mechanisms responsible for neuronal degeneration and axon regeneration while maintaining a consistent focus on clinically relevant scenarios and therapies that will translate into effective vision restoration treatments.

Anna La Torre, PhD
Associate Professor, Department of Cell Biology and Human Anatomy
School of Medicine, University of California, Davis
Davis, CA
Dr. La Torre’s laboratory focuses on generating retinal ganglion cells from stem cells to enhance axonal growth and cell survival and ultimately, to use these cells as donor cells for cell replacement therapies and disease modeling.

Derek Welsbie, MD, PhD
Assistant Professor of Ophthalmology, San Diego Shiley Eye Institute
University of California, San Diego
The Welsbie lab focuses on identifying genes that are causally involved in retinal ganglion cell death, degeneration, and regeneration, as well as developing new neuroprotective drug therapies for retinal ganglion cells.

 DONATE NOW
DONATE NOW