What is Glaucoma?
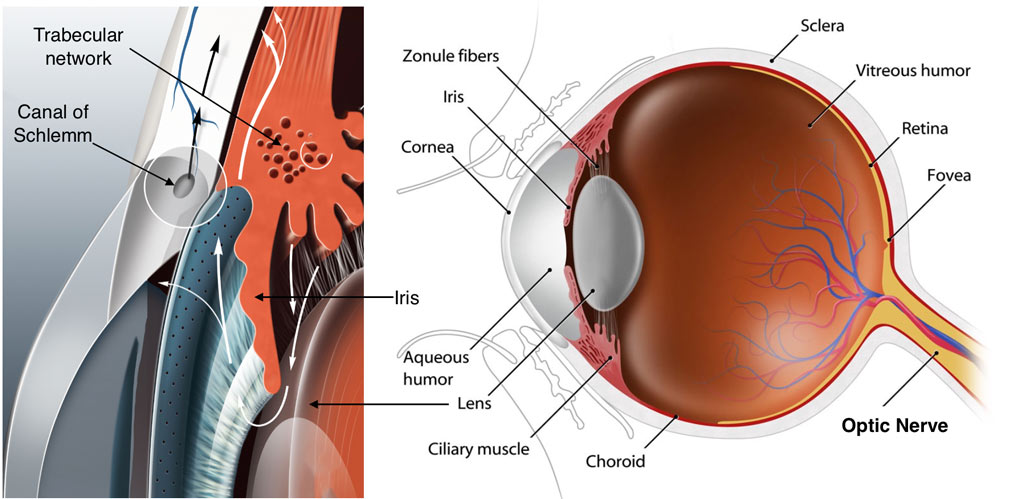
Glaucoma is a leading cause of blindness in the U.S. and worldwide. The most important type of glaucoma is primary open angle glaucoma (POAG). Elevated intraocular pressure poses the most important risk for POAG. A normal eye contains a fluid called aqueous humor, and the eye maintains pressure by balancing aqueous humor production and drainage. In POAG, the drainage system clogs, causing pressure to build up in the eye (usually >21 mmHg). This high pressure will cause permanent damage to a group of cells in the retina at the back of the eye, and these cells send visual signals to the brain. Pressure dependent damage to these cells leads to permanent visual loss. It is now clear that the blockage in the drainage system is due to abnormal changes in the trabecular meshwork (TM). This tissue functions like a valve that fine regulates drainage rate and eye pressure.
Current anti-glaucoma eye drops are initially effective in lowering eye pressure. However, these eye drops tend to become less effective after prolonged use because glaucoma continues to damage the TM. Many patients may need anti-glaucoma surgery because eye drops can no longer control eye pressure after several years. Surgeries, although serve well in many patients, have potential complications. Surgeries may also failure overtime due to too much scar formation or other conditions.
The problem with current eye drops is that they do not correct the disorder inside the TM. Instead, they lower eye pressure by either decreasing aqueous humor refill (such as Timolol). They can also open up another drainage route that does not involve the TM (such as Xalatan). Although these eye drops lower eye pressure, the disease condition inside the TM still exists and keeps getting worse. As glaucoma progresses, drainage through the TM worsens, and eye drops can no longer control rising eye pressure.
What is Our Strategy for Treating Glaucoma?
As described above, the ideal treatment for POAG is to correct the problem in the drainage system, especially the TM tissue. With those problems corrected, the drainage rate will return to normal and so will the eye pressure. Many studies have shown that abnormal levels of cell factors, a group of small proteins used by cells for communication, can damage the TM in various experimental models. Therefore, doctors often call them POAG-associated factors. More importantly, the damage found in those models are very similar to those found in POAG patients. Ideally, doctors can heal the drainage system, lower eye pressure, and protect POAG patients’ vision by identifying, understanding, and normalizing elevated factors.
What Do We Study?
This study focuses on:
- Which cell factors are elevated simultaneously in POAG TM. We are investigating if there is a specific pattern/combination of these factors. For example, for some POAG patients, factors A and B may coexist; while for other patients, factors A and C may coexist.
- What causes the elevation of these factors in the POAG eye. If there is a specific pattern or combination of certain factors, we can compare the modification of those factors’ genes, a mechanism called epigenetics. This comparison will tell us if epigenetics play a role in regulating POAG-associated factors.
How Do We Study?
We are collecting aqueous humor samples from cataract surgeries and anti-glaucoma surgeries. We also collect TM tissues excised from anti-glaucoma surgeries as well as donor eyes (because no TM tissue is removed during cataract surgeries). Samples are provided by Dr. Fellman and his colleagues at the Glaucoma Associates of Texas. All our protocols have been reviewed and approved by the UNTHSC institutional review board, a committee that evaluates research involving human subjects and ensures those subjects are maximally protected.
The level of the POAG-associated factors will be compared between samples collected from cataract surgeries (as a control) and from anti-glaucoma surgeries. The modification of genes will also be compared between TM tissues collected from donor eyes (as a control) and from anti-glaucoma surgeries. We will use special equipment in the laboratory to accurately measure those factors and potential differences in gene modifications. The results will be analyzed using statistical methods to determine whether our findings are conclusive or “random.”
What Do We Expect to See?
Upon the successful completion of the study, we expect to have determined the pattern of POAG-associated factors in the TM and the difference in DNA modification. This will provide us with a strong scientific basis to develop new disease modifying therapies to better treat glaucoma patients and more effectively preserve their vision.

Journal Article

Weiming Mao, PhD
Dr. Mao began this study at the North Texas Eye Research Institute (NTERI) in Fort Worth, Texas. He has since joined the Department of Ophthalmology at the Indiana University School of Medicine and is currently:

 DONATE NOW
DONATE NOW